Year :
2018
Title :
Chemistry
Exam :
WASSCE/WAEC MAY/JUNE
Paper 1 | Objectives
21 - 30 of 50 Questions
| # | Question | Ans |
|---|---|---|
| 21. |
The number of Hydrogen ions in 1.0\(dm^{3}\) of 0.02\(moldm^{-3}\) tetraoxosulphate(VI) acid is \([N_{A} = 6.02 \times 10^{23}]\) A. \(1.2 \times 10^{22}\) B. \(1.2 \times 10^{23}\) C. \(2.4 \times 10^{22}\) D. \(2.4 \times 10^{23}\) Detailed Solution\(1mole = 6.02 \times 10^{23} ions\)\(0.02moldm^{-3} = 0.02 \times 6.02 \times 10^{23}\) =\(0.1204 \times 10^{23} = 1.204 \times 10^{22}\) \(\approxeq 1.2 \times 10^{22}\) |
|
| 22. |
The most suitable substance for putting out petrol fire is A. water B. carbon(IV) oxide C. fire blanket D. sand Detailed SolutionIn order to extinguish a petrol fire, you have to cut off the oxygen. This can be done by using carbon dioxide gas to dilute the oxygen available and stop the burning. |
|
| 23. |
The following factors would contribute to environmental pollution except A. production of ammonia B. manufacture of cement C. photosynthesis D. combustion Detailed SolutionPhotosynthesis is the process of food-making in plants, it doesn't have any by-products that pose a threat to the environment. Infact, the plants use our waste product of respiration (Carbon dioxide) and uses it for the photosynthetic process and gives off oxygen as by-product. |
|
| 24. |
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants B. vigorous stirring of the reaction mixture C. presence of a catalyst D. change in concentration of the reactants Detailed SolutionThe position of equilibrium will shift under the effects of changes in temperature, pressure and concentration. |
|
| 25. |
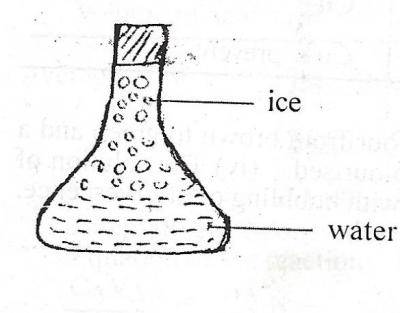 The diagram above illustrates a conical flask containing water and ice. Which of the following is correct about the diagram? A. The water is at lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point Detailed SolutionHeat energy near the ice gets absorbed into the ice. The energy breaks the bonds of the ice, causing it to melt into liquid water. On other words, when ice melts, the energy gets absorbed into the water. |
|
| 26. |
Which of the following statements best explains the difference between a gas and a vapour? A. Unlike gases, vapours are liquids at room temperature B. Unlike gases, vapour can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases Detailed SolutionVapour is the equilibrium state between the gases and liquid, and it can bounce back to its original form, liquid with constant temperature and pressure exerted on it. |
|
| 27. |
Consider the following reaction equation: \(2HCl + Ca(OH)_{2} \to CaCl_{2} + H_{2}O\). What is the volume of 0.1\(moldm^{-3}\) HCl that would completely neutralize 25\(cm^{3}\) of 0.3\(moldm^{-3}\) Ca(OH)\(_{2}\)? A. 150\(cm^{3}\) B. 75\(cm^{3}\) C. 30\(cm^{3}\) D. 25\(cm^{3}\) Detailed SolutionUsing the formula, \(\frac{C_{A}V_{A}}{C_{B}V_{B}} = \frac{N_{A}}{N_{B}}\), we have\(\frac{0.1 \times V_{A}}{0.3 \times 25} = \frac{2}{1}\) \(V_{A} = \frac{0.3 \times 25 \times 2}{0.1 \times 1}\) \(V_{A} = 150cm^{3}\) |
|
| 28. |
\(Cu\) and \(HNO_{3}\) are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively B. conductivity and corrosiveness respectively C. melting point and reduction respectively D. electronegativity and solubility respectively Detailed SolutionCopper is not active enough to replace hydrogen in an acid. Also, nitric acid is a strong oxidizing agent which quickly oxidises hygrogen to water. |
|
| 29. |
Which of the following CANNOT be an empirical formula? A. \(CH\) B. \(CH_{2}\) C. \(P_{2}O_{5}\) D. \(N_{2}O_{4}\) Detailed SolutionThe empirical formula is always in the ratio of the smallest positive integer, hence, \(N_{2}O_{4}\) has an empirical formula \(NO_{2}\). |
|
| 30. |
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity B. boiling point C. mass D. colour Detailed SolutionAny pure substance will have a specific melting and boiling point. The presence of impurities alters the boiling point of benzene. |
| 21. |
The number of Hydrogen ions in 1.0\(dm^{3}\) of 0.02\(moldm^{-3}\) tetraoxosulphate(VI) acid is \([N_{A} = 6.02 \times 10^{23}]\) A. \(1.2 \times 10^{22}\) B. \(1.2 \times 10^{23}\) C. \(2.4 \times 10^{22}\) D. \(2.4 \times 10^{23}\) Detailed Solution\(1mole = 6.02 \times 10^{23} ions\)\(0.02moldm^{-3} = 0.02 \times 6.02 \times 10^{23}\) =\(0.1204 \times 10^{23} = 1.204 \times 10^{22}\) \(\approxeq 1.2 \times 10^{22}\) |
|
| 22. |
The most suitable substance for putting out petrol fire is A. water B. carbon(IV) oxide C. fire blanket D. sand Detailed SolutionIn order to extinguish a petrol fire, you have to cut off the oxygen. This can be done by using carbon dioxide gas to dilute the oxygen available and stop the burning. |
|
| 23. |
The following factors would contribute to environmental pollution except A. production of ammonia B. manufacture of cement C. photosynthesis D. combustion Detailed SolutionPhotosynthesis is the process of food-making in plants, it doesn't have any by-products that pose a threat to the environment. Infact, the plants use our waste product of respiration (Carbon dioxide) and uses it for the photosynthetic process and gives off oxygen as by-product. |
|
| 24. |
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants B. vigorous stirring of the reaction mixture C. presence of a catalyst D. change in concentration of the reactants Detailed SolutionThe position of equilibrium will shift under the effects of changes in temperature, pressure and concentration. |
|
| 25. |
 The diagram above illustrates a conical flask containing water and ice. Which of the following is correct about the diagram? A. The water is at lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point Detailed SolutionHeat energy near the ice gets absorbed into the ice. The energy breaks the bonds of the ice, causing it to melt into liquid water. On other words, when ice melts, the energy gets absorbed into the water. |
| 26. |
Which of the following statements best explains the difference between a gas and a vapour? A. Unlike gases, vapours are liquids at room temperature B. Unlike gases, vapour can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases Detailed SolutionVapour is the equilibrium state between the gases and liquid, and it can bounce back to its original form, liquid with constant temperature and pressure exerted on it. |
|
| 27. |
Consider the following reaction equation: \(2HCl + Ca(OH)_{2} \to CaCl_{2} + H_{2}O\). What is the volume of 0.1\(moldm^{-3}\) HCl that would completely neutralize 25\(cm^{3}\) of 0.3\(moldm^{-3}\) Ca(OH)\(_{2}\)? A. 150\(cm^{3}\) B. 75\(cm^{3}\) C. 30\(cm^{3}\) D. 25\(cm^{3}\) Detailed SolutionUsing the formula, \(\frac{C_{A}V_{A}}{C_{B}V_{B}} = \frac{N_{A}}{N_{B}}\), we have\(\frac{0.1 \times V_{A}}{0.3 \times 25} = \frac{2}{1}\) \(V_{A} = \frac{0.3 \times 25 \times 2}{0.1 \times 1}\) \(V_{A} = 150cm^{3}\) |
|
| 28. |
\(Cu\) and \(HNO_{3}\) are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively B. conductivity and corrosiveness respectively C. melting point and reduction respectively D. electronegativity and solubility respectively Detailed SolutionCopper is not active enough to replace hydrogen in an acid. Also, nitric acid is a strong oxidizing agent which quickly oxidises hygrogen to water. |
|
| 29. |
Which of the following CANNOT be an empirical formula? A. \(CH\) B. \(CH_{2}\) C. \(P_{2}O_{5}\) D. \(N_{2}O_{4}\) Detailed SolutionThe empirical formula is always in the ratio of the smallest positive integer, hence, \(N_{2}O_{4}\) has an empirical formula \(NO_{2}\). |
|
| 30. |
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity B. boiling point C. mass D. colour Detailed SolutionAny pure substance will have a specific melting and boiling point. The presence of impurities alters the boiling point of benzene. |