Year :
1990
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 49 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
If 10.8 g of silver is deposited in a silver coulometer volume of oxygen liberated is? A. 0.56 dm3 B. 5.60 dm3 C. 11.20 dm3 D. 22.40 dm3 Detailed SolutionAg+ + e-H = 108 g Ag = 0.11 10.8 Ag 4OH → O2 + 2H2O + 4e 4F ↔ 1mole O2 4F = 22.4 dm3 χ 0.1 = 0.56 dm3 |
|
| 42. |
0.1 faraday of electricity deposited 2.95 g of nickel during electrolysis of an aqueous solution. A. 0.20 B. 0.30 C. 0.04 D. 5.87 Detailed Solution0.1F ↔ 2.95 g Ni = 0.4F ↔ 11.8 g NiNo of moles = (11.8)/(58.2) = 0.20 moles |
|
| 43. |
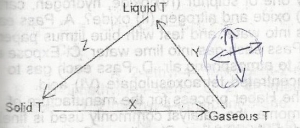 Changes in the physical in the scheme above.The letter X,Y and Z respectively represent A. sublimation, condensation and freezing B. sublimation, vapourization and solidification C. freezing, condensation and sublimation D. evaporation, liquefaction and solidification |
A |
| 44. |
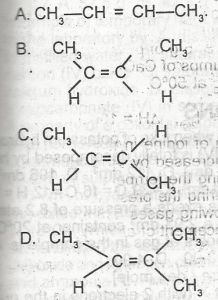 The structure of cis-2-butene is A. A B. B C. C D. D |
B |
| 45. |
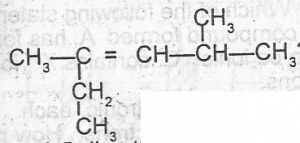 What is the IUPAC name for the hydrocarbon A. 2-ethyl-4-methylpent-2-ene B. 3,5-dimethylhex-3-ene C. 2,4-dimethylhex 3-ene D. 2-methyl-4-ethylpent-3-ene |
A |
| 46. |
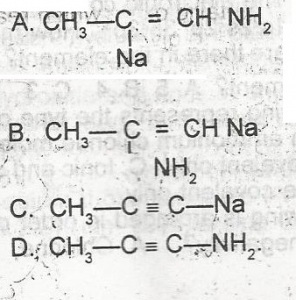 CH3-C = CH \(\frac{Na}{liq NH_3}\)> P, Compound P, in the above reaction, is A. A B. B C. C D. D |
B |
| 47. |
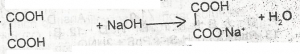 The above reaction is an example of A. a displacement reaction B. a neutralization reaction C. an elimination reaction D. saponification |
B |
| 48. |
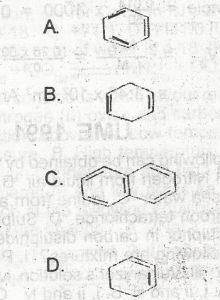 Which of the following compounds represents the polymerization product of ethyne? A. A B. B C. C D. D |
A |
| 49. |
The Avogadro number of 24g of magnesium is the same as that of A. 1g of hydrogen molecules B. 16g of oxygen molecules C. 32g of oxygen molecules D. 35.5g of chlorine molecules Detailed Solution24mg = 1 mole of magnesium and32g of O2 = mole of O2 |
| 41. |
If 10.8 g of silver is deposited in a silver coulometer volume of oxygen liberated is? A. 0.56 dm3 B. 5.60 dm3 C. 11.20 dm3 D. 22.40 dm3 Detailed SolutionAg+ + e-H = 108 g Ag = 0.11 10.8 Ag 4OH → O2 + 2H2O + 4e 4F ↔ 1mole O2 4F = 22.4 dm3 χ 0.1 = 0.56 dm3 |
|
| 42. |
0.1 faraday of electricity deposited 2.95 g of nickel during electrolysis of an aqueous solution. A. 0.20 B. 0.30 C. 0.04 D. 5.87 Detailed Solution0.1F ↔ 2.95 g Ni = 0.4F ↔ 11.8 g NiNo of moles = (11.8)/(58.2) = 0.20 moles |
|
| 43. |
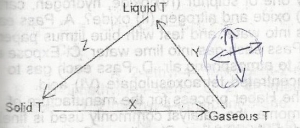 Changes in the physical in the scheme above.The letter X,Y and Z respectively represent A. sublimation, condensation and freezing B. sublimation, vapourization and solidification C. freezing, condensation and sublimation D. evaporation, liquefaction and solidification |
A |
| 44. |
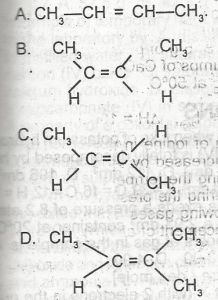 The structure of cis-2-butene is A. A B. B C. C D. D |
B |
| 45. |
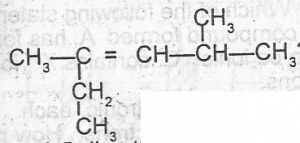 What is the IUPAC name for the hydrocarbon A. 2-ethyl-4-methylpent-2-ene B. 3,5-dimethylhex-3-ene C. 2,4-dimethylhex 3-ene D. 2-methyl-4-ethylpent-3-ene |
A |
| 46. |
 CH3-C = CH \(\frac{Na}{liq NH_3}\)> P, Compound P, in the above reaction, is A. A B. B C. C D. D |
B |
| 47. |
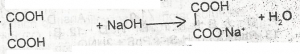 The above reaction is an example of A. a displacement reaction B. a neutralization reaction C. an elimination reaction D. saponification |
B |
| 48. |
 Which of the following compounds represents the polymerization product of ethyne? A. A B. B C. C D. D |
A |
| 49. |
The Avogadro number of 24g of magnesium is the same as that of A. 1g of hydrogen molecules B. 16g of oxygen molecules C. 32g of oxygen molecules D. 35.5g of chlorine molecules Detailed Solution24mg = 1 mole of magnesium and32g of O2 = mole of O2 |