Year :
1992
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 50 of 51 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
Which of the following are isomers? A. Ethanol and dimethyl ether B. Benzene and methybenzene C. Ethanol and propanone D. Trichloromethane and tetrachloromethane |
A |
| 42. |
The functional group present in an organic compound which gives bubbles on treatment with a saturated solution of NaHCO3 is? A. hydroxyl group B. carboalkoxyl group C. carbonyl group D. carboxyl group |
D |
| 43. |
An organic compound containing 40.1% carbon and 6.6% hydrogen has an empirical formula of? A. C2H4O2 B. C2H3O2 C. CH2O D. CH3O Detailed SolutionCarbon Hydrogen40.1..........................6.67 (3.342) / (3.342).............(6.67) / (3.342) 1 1.9958 1 Ω 2 CH2O |
|
| 44. |
The characteristics reaction of carbonyl compounds is? A. substitution B. elimination C. addition D. saponfication |
C |
| 45. |
Alkanals can be differentiated from alkanones by reaction with? A. 2,4-dinitrophenylhydrazine B. hydrogen cyanide C. sodium hydrogen sulphite D. Tollen's reagent |
D |
| 46. |
An example of a polysaccharides is? A. —dextrose B. mannose C. glucose D. starch |
D |
| 47. |
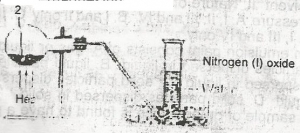 In the experiment above. Z can be A. a solution of sodium dioxonitrate (lll) and ammonium chloride B. a solution of lead trioxnitrate (V) C. a solution of sodium trioxonitrate (V) and ammonium chloride D. concentrated tetraoxosulphate (VI) acid and sodium trioxonitrate (V) |
C |
| 48. |
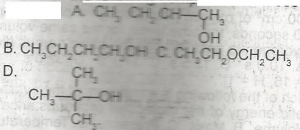 Which of the following compounds is a secondary alkanol? A. A B. B C. C D. D |
A |
| 49. |
A given mass of gas occupies 2 dm2 at 300k. At what temperature will its volume be doubled keeping the pressure constant? A. 400k B. 480k C. 550k D. 600k |
D |
| 50. |
Which of the following is a measure of the average kinetic energy of the molecules of a substance? A. volume B. mass C. pressure D. temperature |
D |
| 41. |
Which of the following are isomers? A. Ethanol and dimethyl ether B. Benzene and methybenzene C. Ethanol and propanone D. Trichloromethane and tetrachloromethane |
A |
| 42. |
The functional group present in an organic compound which gives bubbles on treatment with a saturated solution of NaHCO3 is? A. hydroxyl group B. carboalkoxyl group C. carbonyl group D. carboxyl group |
D |
| 43. |
An organic compound containing 40.1% carbon and 6.6% hydrogen has an empirical formula of? A. C2H4O2 B. C2H3O2 C. CH2O D. CH3O Detailed SolutionCarbon Hydrogen40.1..........................6.67 (3.342) / (3.342).............(6.67) / (3.342) 1 1.9958 1 Ω 2 CH2O |
|
| 44. |
The characteristics reaction of carbonyl compounds is? A. substitution B. elimination C. addition D. saponfication |
C |
| 45. |
Alkanals can be differentiated from alkanones by reaction with? A. 2,4-dinitrophenylhydrazine B. hydrogen cyanide C. sodium hydrogen sulphite D. Tollen's reagent |
D |
| 46. |
An example of a polysaccharides is? A. —dextrose B. mannose C. glucose D. starch |
D |
| 47. |
 In the experiment above. Z can be A. a solution of sodium dioxonitrate (lll) and ammonium chloride B. a solution of lead trioxnitrate (V) C. a solution of sodium trioxonitrate (V) and ammonium chloride D. concentrated tetraoxosulphate (VI) acid and sodium trioxonitrate (V) |
C |
| 48. |
 Which of the following compounds is a secondary alkanol? A. A B. B C. C D. D |
A |
| 49. |
A given mass of gas occupies 2 dm2 at 300k. At what temperature will its volume be doubled keeping the pressure constant? A. 400k B. 480k C. 550k D. 600k |
D |
| 50. |
Which of the following is a measure of the average kinetic energy of the molecules of a substance? A. volume B. mass C. pressure D. temperature |
D |